Snapshots Behind The Scenes


Some Areas of Research & Development

One of Nick Kalavrezos' interests lies in maximising the use of clinical robots to improve and develop micro-surgical procedures. The continuous rapid development of surgeon-guided robots demands that our surgical techniques must evolve to exploit their potential.
Clinical robots can operate through a very small incision and bring to bear stereoscopic cameras, endoscopes, lights and a multiplicity of surgical implements to the internal operating area. They are a major factor in reducing the degree of surgery and its impact overall.
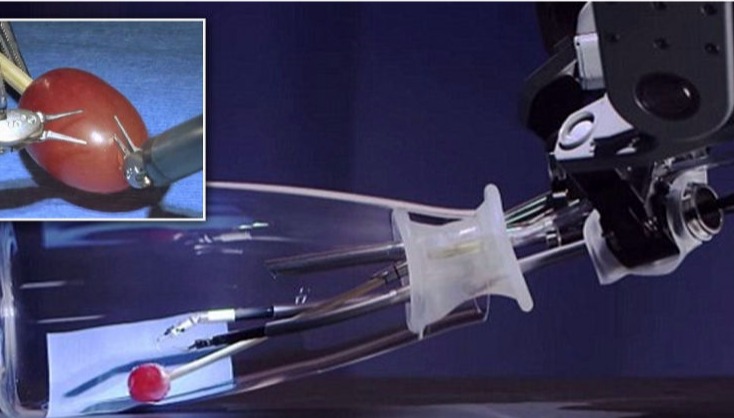

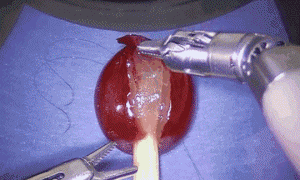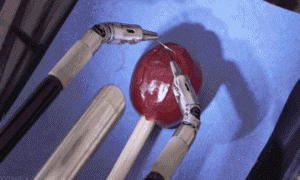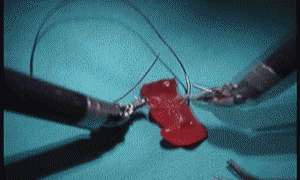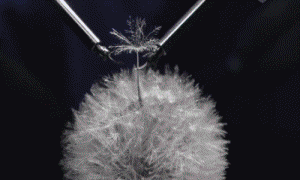
Above: Major surgery on grapes, jelly-babies and dandelions!
Surgeon-guided robots such as the Da Vinci Clinical Robot are a major tool in minimally invasive surgery.
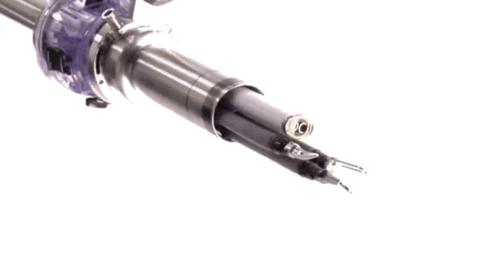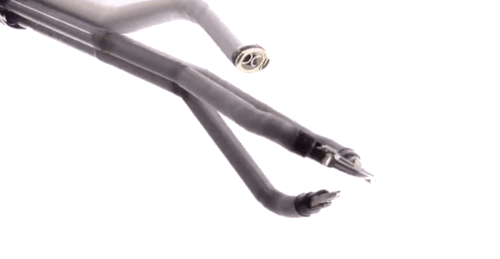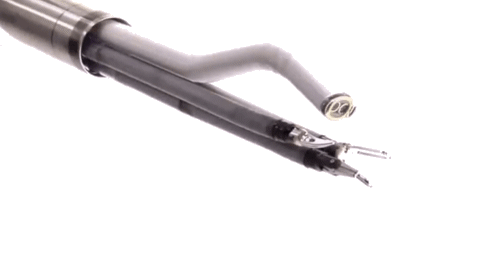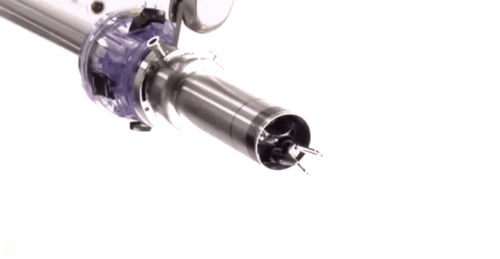


Until quite recently, surgeons had to employ open surgery in situations where access was difficult. For example they were unable to reach the back of the mouth and throat without major incisions and causing inevitable trauma to the jaw.
Today, for benign (noncancerous) conditions and early stage cancers, we can use minimally invasive techniques with robots which work through small incisions to reach areas that have traditionally been inaccessible without major surgery.
Minimally invasive surgery contributes to:
• A shorter hospital stay
• A faster recovery
• Less visible scarring
• Less chance of infection after surgery
• Less chance of disfigurement
• Fewer or minimal external incisions
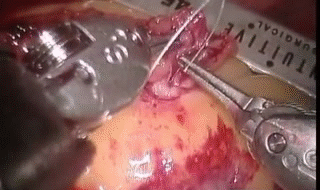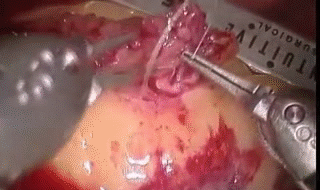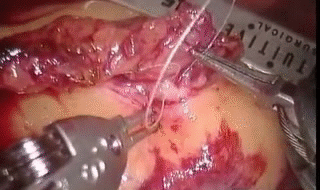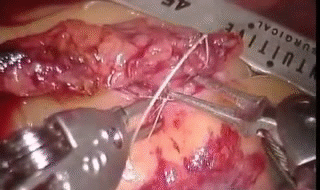
Nick is a member of the Face Transplant Group based at the Royal Free Hospital, aiming to perform the first face transplant in the UK. The research in regenerative medicine and tissue engineering (opposite) and the constant development of micro-surgery is increasing the likelihood that fully successful transplants will be carried out in the near future.
Facial reconstruction surgery is used to restore a person’s appearance to a relatively normal condition after a traumatic injury that has severely damaged the face and appearance. Such an injury may remove part or all of the face.

Computer technology is constantly offering new ways to plan complex reconstructive surgery. We can take advantage of a virtual environment to view the area in depth, plan the procedure accurately and rehearse it virtually.
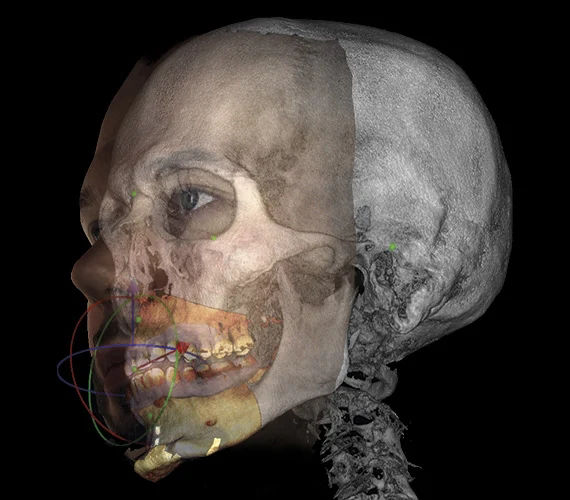
Image courtesy of Planmeca
This technology is reducing the need for exploratory surgery and is translating into more predictable and better outcomes for our patients. It also gives the patient a better understanding and greater confidence.
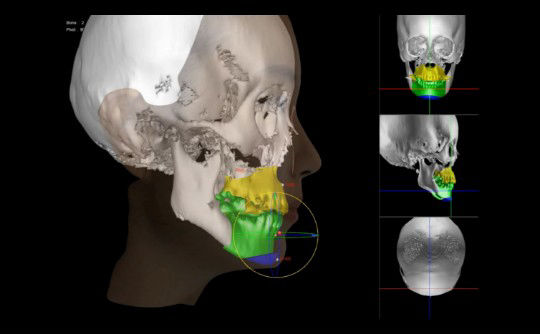
Image courtesy of Morpheus3D
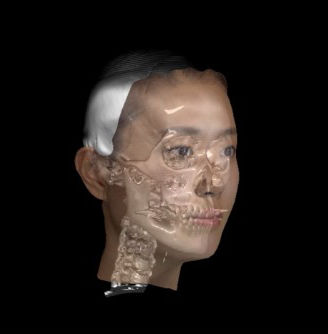
Image courtesy of Morpheus3D
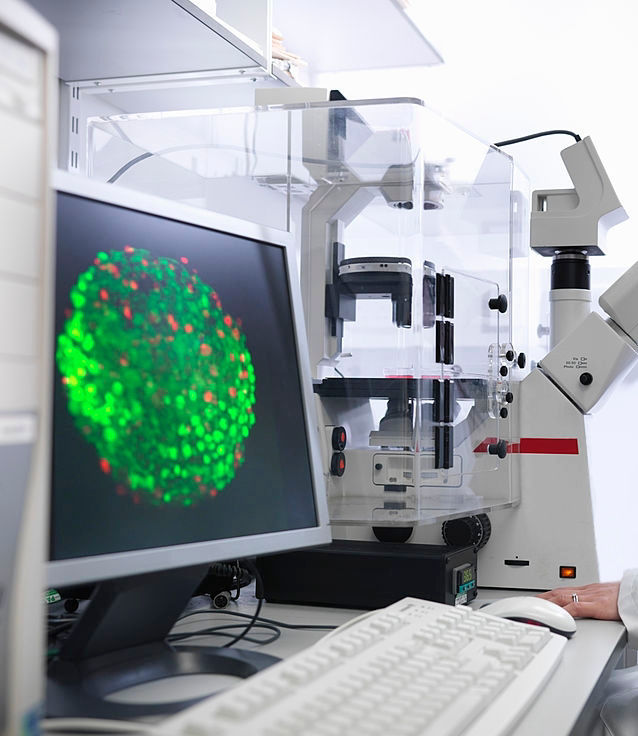
Nick Kalavrezos has joint research projects in the Nanotechnology Lab of UCLH on tissue engineered bio-scaffolding, aiming to replace defects and tissue loss in the face and neck with laboratory-grown tissue flaps.
What is tissue engineering? Tissue engineering uses human stem cells to replace or repair damaged tissue by cultivating the cells in the laboratory to produce new replacement tissue. It brings together genetic engineering, stem cell research, materials science, software design, computing, engineering methods and 3D printing. It is the next chapter in organ transplantation and offers the potential to routinely replace damaged or diseased organs with replacements grown from the patient's own stem cells. It is expected to remove the need for donor organs while largely eliminating rejection problems.
Stem cells - what are they? To over-simplify, they can be thought of as 'primitive' or 'unprogrammed' cells. They underpin our body's internal repair system and can divide (mitosis) many times to make more stem cells or replace any specific type of cell. They contain the genetic "master code" for all the cell types in our bodies and can produce whichever type of cell is needed, such as bone cells, cheek muscle cells or cartilage cells.
We have our own supply of stem cells which can be harvested and cultivated in the laboratory to develop into specific types of tissue which can then be transplanted back to the trauma site in the patient. 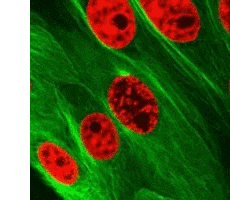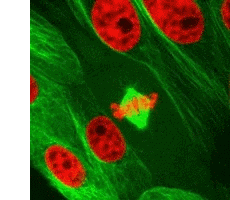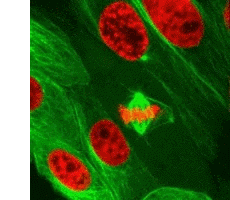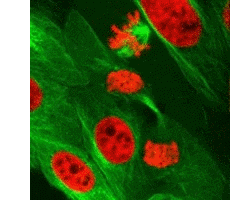
Right: Cell division (mitosis)
Foetal cells in a new embryo are a good example of stem cell function. As the embryo grows, they divide and differentiate into heart, brain, bone or whatever type of cell is needed.
After cultivating the patient's stem cells in the laboratory, they are re-introduced to the patient in different ways. The cells may be injected or if cultivated as tissue, applied directly to the patient as a tissue flap (burns or restorative facial surgery).
The potential for stem cells extends to regrowing complex organs such as tongue, heart, liver and kidney on bio scaffolds (fabricated shapes). At this time (2021) research is within sight of being able replace organs as a matter of routine.
Growing replacement tissue on bio-scaffolds
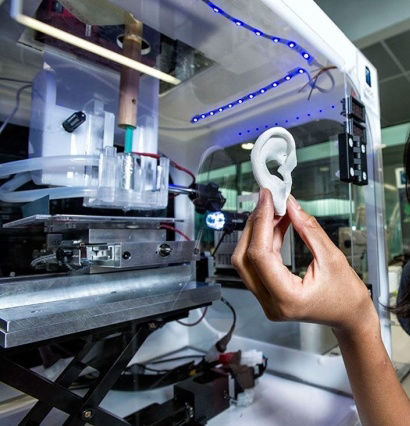
Scaffolds are used as 'shape guides' when whole organs or larger areas of patient tissue need to be grown to replace a missing area or organ - for example an ear. The scaffold is a shaped, porous mesh which is produced with a 3D printer. It can best be visualised as a wide-mesh net that has been loosely rolled, folded and moulded into shape.
Once produced, the scaffold is permeated with a growth factor (cell nutrient solution) containing stem cells which replicate and grow over and through the scaffold mesh. Eventually the scaffold is entirely covered with new living tissue. The scaffold itself is normally degradable and is usually absorbed harmlessly by the body. The new ear is attached to the patient using (difficult) micro-vascular surgical methods.
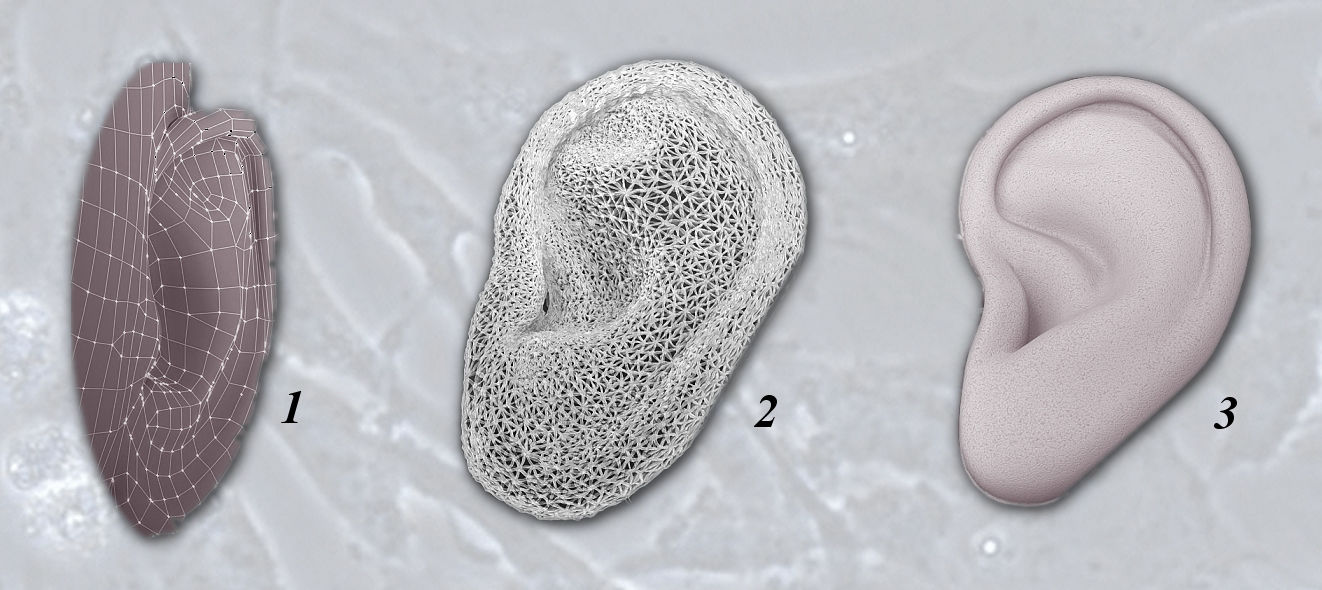
 Complex scaffolds
Complex scaffolds
1. A three dimensional computer scan of the ear is made. 2. A 3D printer reads the large data file from the scan to print out the scaffold 3. The scaffold is 'seeded' with stem cells in a growth factor suspension. They multiply to the shape of the scaffold. Right: 3D printed scaffold and closeup.
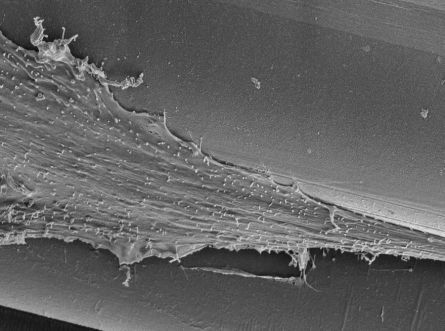
The new ear (3)is re-attached to the patient using micro-vascular surgery. The artificial printed scaffold material is eventually absorbed by the body. Below: Stem cells growing within a scaffold mesh
 Right: Simple flat tissue scaffolds
Right: Simple flat tissue scaffolds
For simpler flat sections that do not need to hold their shape, 3D scaffolds can be made by extruding gel in the shape of a flat matrix.. The gel itself contains the stem cells and nutrients.
These flat matrices can be used to produce flaps of tissue to treat burns, replace scars, or replace tissue lost through surgery. Micro-vascular surgery is required to connect nerves and blood vessels between the engineered tissue flap and the patient's body at the site of the injury.
Within our practice these tissue flaps are now removing the need to use tissue harvested from donor sites on the patients body for facial or neck restoration.
Tissue engineering also allows us to 'copy' malignant tumours using Crispr gene editing and grow them in the laboratory. Different treatment options can then be safely tested on the laboratory-grown tumour at greatly reduced risk to the patient.
What is micro-vascular flap surgery?
Micro-vascular flap surgery is very often a crucial part of facial reconstructive surgery. It has usually meant removing tissue from one part of the body to repair damaged or missing tissue elsewhere. If, for example, the patient has lost part of their cheek through cancer, a flap of tissue has been taken from the patient’s abdomen or buttock and transferred to the face. Some of the difficulties have always been to minimise morbidity (damage) at the donor site and to ensure blood supply and nerve function at the target site.
Flap surgery demands a range of high-end skills, including micro-vascular surgery to reconnect very small blood vessels and nerves at the target site.
Nick is refining flap surgery to avoid some of the problems. The tissue-engineered micro-vascular flaps grown on the flat matrices (shown above) have their own nerve and blood supplies and remove the need for harvesting tissue from donor sites on the patients's own body.
The production of these flat, simpler-shaped tissue flaps is not held up by the research that is still needed to produce larger scaffolds with complex shapes. Their production is not so dependant on materials science, or the hardware and software needed to produce scaffolds for ears, whole faces or complete organs.
Our current research is addressing several challenges:
1) Difficulties with scaffolds
Scaffolds with complex shapes such as a face are difficult to produce, mainly because they must be sufficiently porous to allow the stem cells and growth factor to permeate through the whole structure. This requirement makes them fragile. Normal 3D printing which produces a solid shape is not suitable because the new stem cells cannot survive on a two dimensional solid surface. The scaffold also needs to be strong, non-toxic, chemically inert and non-reactive within the body.
In engineering terms these requirements present challenges. Materials scientists are researching the ideal material for scaffold production. The requirements are also forcing the rapid evolution of advanced 3D printers and CAD software to control them. The scanning of complex shapes and printing them as a fine, three dimensional porous mesh produces massive data files. Quantum computing is part of the research effort.
For these reasons and others, complex structures such as a face or a human heart have not yet been produced (early 2021).
Our facial transplant program at the Royal Free Hospital is dependant on this research moving forward.
However there is a sense of certainty that many of the problems can be solved in the very near future.
2) Challenges with stem cell research
We don't yet understand enough about cell behaviour. Stem cells contain the genetic code for all types of cells in the body and they replicate into the type of cell that is needed at their specific location. We know quite a lot about the role of RNA (ribo-nucleic acid) in determining the role of the replicated daughter cell that is produced by a stem cell. As it divides, the stem cell deactivates all the DNA code in the new daughter cell except for the fraction of code that the new cell needs to function as a bone cell, muscle cell, or whatever cell type is needed. (epigenetic silencing).
But we don't fully understand how the switching is activated, or what controls the timing of cell division (mitosis). We are also trying to understand how the stem cell "knows" it has been placed in a cheek and therefore must replicate more cheek cells and not - for example - bone cells or something else! Also, how does a stem cell "know" when to stop replicating?
Uncontrolled cell replication is the main characteristic of mutant cells which form cancerous tumours. Yet minute by minute the cell-division process in our bodies runs almost perfectly, tens of billions of times a day for most of our lives.
The picture is very far from complete. But our current progress in starting to understanding these extraordinary processes drives and motivates everybody involved. The awareness of where it is leading and the possibilities that can be glimpsed make this research the most productive and exciting area to work in.
It is having a major impact on patient care. We see the results daily.
Free AI Website Creator
© Copyright 2021/26 N Kalavrezos
London Head & Neck Ltd